

The filtrate was centrifuged at 3000 rpm and freeze-dried, yielding Pd2+ dispersed on GO (Pd2+/GO).
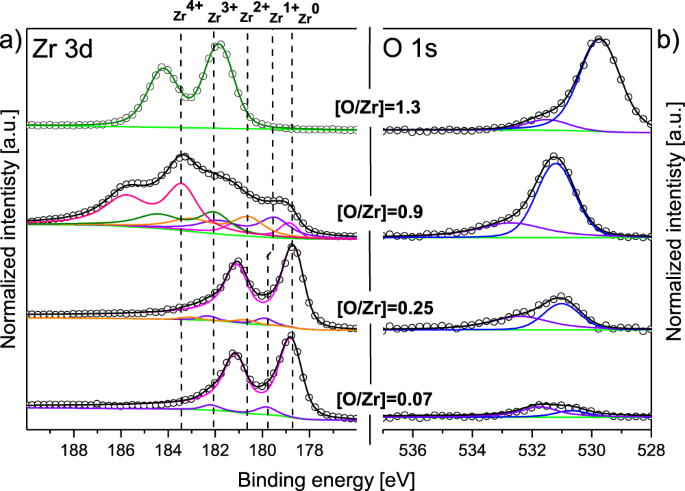
The suspension was filtered and washed with distilled water several times. The resulting suspension was sonicated for 5 min and stirred for 24 h to promote the intercalation of Pd2+ on the GO surface. Typically, GO (0.9 g) was mixed with Pd(OAc)2 (0.21 g) in 90 mL of distilled water, yielding a homogenous, dark yellow. Pd NPs dispersed on graphene (Pd/G) and PdO NPs on graphene oxide (PdO/GO) were prepared through an impregnation method combined with thermal treatments with H2 and O2 gases, respectively. The final product (graphene oxide) was freeze-dried and stored in a vacuum desiccator until further use. Then, the suspension was filtered and washed with a 0.1 M HCl solution and distilled water, and then centrifuged at 3,000 rpm. The whole reaction mixture was carefully poured into a 5 L flask in an ice bath and H2O2 (30%) was added until gas was no longer detected. After 2 h, the suspension was removed from the ice bath and warmed to 35 ℃. Graphene oxide (GO) was prepared by the oxidation of graphite powder with H2SO4/KMnO4, according to the method of Hummers and Offeman.20īriefly, graphite (2.0 g) was added to concentrated H2SO4 (50 mL) in an ice bath, and NaNO3 (1.0 g) and KMnO4 (7.0 g) were slowly added under continuous stirring. However, the dispersion and size distribution of the Pd and PdO NPs are critical for high catalytic efficiency. We found that the oxidation states of Pd and graphene did not significantly influence the catalytic performance. Here, we demonstrate that the Pd and PdO NPs can be well dispersed on graphene and GO, respectively, and that these NPs act as efficient catalysts in the C-C coupling reactions. Concurrently, we have characterized their morphological and electronic structures in order to understand how the size distribution and oxidative states of the Pd NPs affect the catalytic efficiency using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM). We have evaluated their catalytic performance in C-C crosscoupling reactions. To clarify these conflicting explanations regarding the performance of the Pd catalysts, we have synthesized graphene and graphene oxide (GO) supported Pd catalysts through an impregnation method combined with thermal treatments. On the other hand, some studies demonstrated that the oxidative states of Pd NPs play an important role in the catalytic activity, and highly dispersed Pd(0) NPs on graphene sheets are required for high catalytic performance.17,19 This implies that residual oxygen functionalities on the surface of graphene are critical for stabilizing the Pd NPs and catalytic efficiency.16 However, it has been claimed that Pd NPs are stable on oxidized graphene sheets and the first layer of the Pd NPs is mainly present as PdOx.

demonstrated that the catalytic activity is strongly influenced by the size distribution of the Pd NPs on graphene sheets, concluding that an even distribution of small Pd NPs is critical for high catalytic performance.15 However, there have been discrepancies in the characterization of the catalytic performance of graphene-based palladium catalysts.
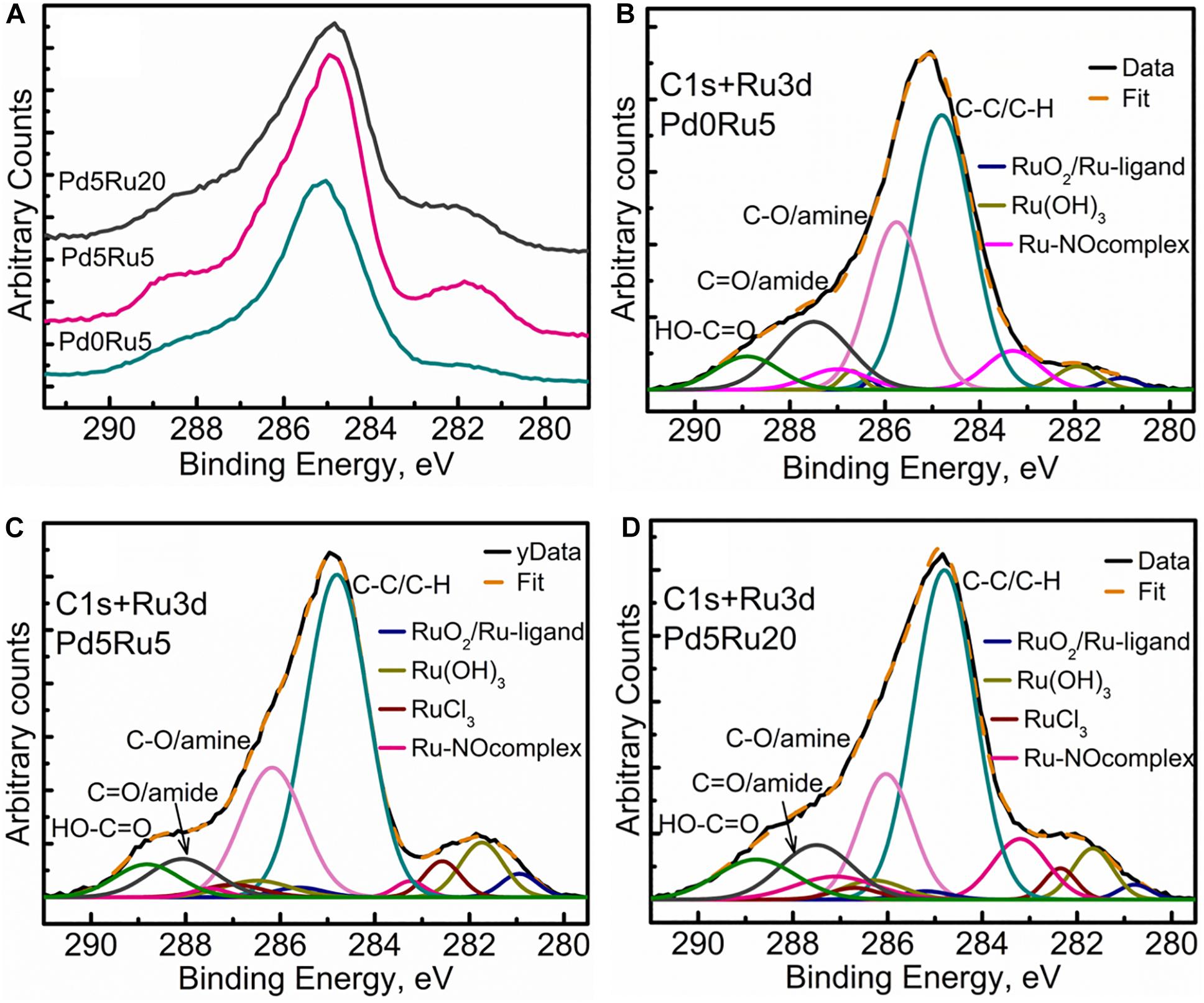
Recently, several research groups have reported that graphene based palladium catalysts possess good activity and selectivity for various C-C coupling reactions.14-18 Palladium (Pd) catalyzed C-C and C-N coupling reactions have been of strategic importance since they offer fast reaction rates, high turnover frequency, good selectivity, and high production yield in various synthetic protocols.12,13 One of the catalytic applications with the graphene supported metal NPs is the area of carbon-carbon (C-C) and carbon-nitrogen (C-N) cross-coupling reactions.9-11 In addition, two-dimensional (2D) sheet of graphene with an extremely high surface-to-volume ratio has great potential, especially as 2D supports for hosting metal nanoparticles (NPs) in heterogeneous catalysis, fuel cells, chemical sensors, and hydrogen storage applications.5-9 Nomber_key:000448Graphene has attracted increasing attention due to its extraordinary electronic, mechanical, and thermal properties, and has motivated the development of new materials for supercapacity, energy production and conversion, molecular electronics and other related devices.1-4


 0 kommentar(er)
0 kommentar(er)
